Lecture notes for March 21, 2018
Limb regeneration in salamanders, and the apical epidermal ridge

Newts and many other kinds of salamanders can regenerate their legs.
No other vertebrates can do this, except partial regeneration of limbs in frog tadpoles before metamorphosis.
Humans can sometimes regenerate the last digit of fingers and toes, IF the wound is NOT stitched up (which is standard practice).
Regenerating newt legs "re-grow" all the bones (i.e. cartilages), muscles, tendons, blood vessels, connective tissue and skin.
In the stump of the cut limb, cartilage, muscle and other cell type become indistinguishable, "dedifferentiating" back to what looks like their early embryonic state, forming a mass of cells called a blastema, in which rapid cell growth and mitosis replaces the lost cells.
Skeletal muscle cells, which are syncytial, subdivide back to individual cells before resuming mitosis.
It would be interesting to know whether this subdivision of multi-nucleate muscle cells
has the same contractile-ring mechanism as the ordinary cytokinesis that follow mitosis.
The myoblasts then undergo many cycles of mitotic cell divisions, and fuse back together.
Grafting of cartilage and muscle labeled with radioactive labeled DNA, and also the use of genetic markers like nucleolus number, consistently show that each cell remains "loyal" to their original differentiated cell type.
In other words the dividing cells in the blastema that had been cartilage cells
re-differentiate only into cartilage,
& the descendants of muscle cells only become muscle.
Most experimenters had not expected this, which make the results even more persuasive.
It also contradicts expectations that regeneration should have the same basic mechanism as the original formation of limbs, except if the original development was by sorting out of cells whose differentiation had already been decided, which nobody considers possible.
Limb regeneration is not thought of as a special case of sorting out by differentiated cells, I guess because there is no deliberate dissociation into single or randomly-mixed cells.
Nevertheless, consider that cartilage cells from which the humerus had previously been constructed separate into what seem to be undifferentiated cells, and then re-differentiate as the radius, ulna digits, wrist bones, as well as replacements for the lost parts of the humerus.
Furthermore, consider that the many muscles of the regenerated leg are made of cells that had been parts of different muscles of the upper limb.
Likewise: skin cells, blood vessels, connective tissue & nerves.
All are replaced by descendants of the same kinds of the cells in the stump of the cut limb.
The Apical Ectodermal Ridge ("AER") is a thickening of the epidermis along an anterior-posterior line at the lateral edge of developing arms, legs and wings. (In birds, the cells of the AER pucker, as if there were a strongly localized contraction at the basal surface of the epithelial cells. [See figures in the lecture notes for March 9th.
AERs form in developing embryos of mammals, birds, reptiles and frogs (but NOT salamanders).
The AER doesn't develop into anything, other than ordinary skin.
Maybe there is some important connection between the fact that salamanders are the only vertebrates that can regenerate legs, and are the (only? tetrapod) vertebrates that don't form apical ectodermal ridges.
Another puzzle is that an epidermal thickening DOES form at the distal tip of regenerating salamander legs. It is not a ridge, however, but a bump.
Teleost fish embryos form AER-like epidermal puckerings at all the places where they will form fins. This includes not just the pectoral and pelvic fins, but also the dorsal, ventral and tail fins. I don't know whether non-teleost fish have such structures.
If the AER cells are a special shape, does their shape mean they are more contractile,
or what? Or is it an evolutionary remnant of fish fin shapes?
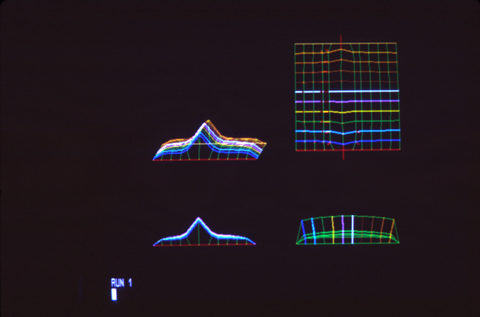
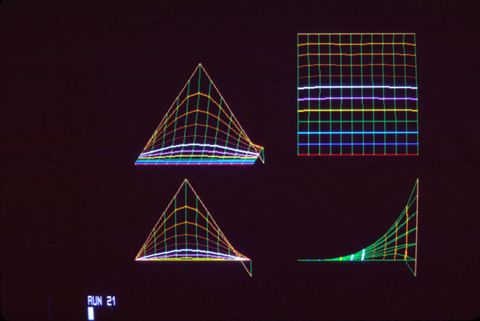
As John Saunders discovered in his PhD thesis (1948), if you cut off the AER, then limb growth will stop. If the AER is removed early in development, then only the upper arm will form; if you cut it off later, the result is that the leg ends near the elbow; and if you cut it off later, then only the "hand" will fail to form.
Grafting an AER from a younger to an older embryo has no effect. Nor does grafting an older AER to a younger limb bud have any effect. Experiments of that kind were done to test the possibility that the AER might gradually changer its inductive power. But the result disproved that idea. Another hypothesis was that the AER might create some kind of diffusion gradient, with distal limb anatomical patterns being induced at long range. But that turned out not to be true, either.
Putting a source of Retinoic Acid (related to vitamin A) on the tip of a developing salamander limb bud can cause proximo-distal development to start over, in the sense of forming another upper-arm, another elbow, another wrist etc.
Putting Retinoic Acid on the tip of a regenerating salamander tail can cause a leg to regenerate (!), instead of a new tail.
Retinoic acid can cause many abnormalities in embryonic development: in other words, it is a teratogen. It was the active ingredient in the strong anti-acne drug "Accutane".
Please notice the important concept that any chemical that alters or controls normal embryonic development is sure to produce birth defects if embryos are exposed to too much of it (and even very low concentrations may be too much).
Increasing retinoic acid concentrations in developing embryos can cause a shift in the anterior borders of the areas where hox genes are transcribed and translated (please don't say "expressed", as almost everyone does, except when describing a specific phenotype). Lots of people say "gene expression" when what they mean is "in situ" staining of some particular gene's mRNA.
It is a popular theory that concentration gradients of retinoic acid in embryonic tissues are what normally controls where Hox genes "are expressed". It may even turn out to be true.
So... the proximo-distal (= medio-lateral) axis of limb bud development is controlled by duration (not concentration) of exposure to some of the fibroblast growth factor proteins, and/or other signals from the AER.
John Saunders also discovered what happens if you graft tissue from the posterior side of developing limb buds to their anterior side. The result is mirror image branching of the distal end of the leg or wing.
For several years, this was said to result from a gradient of retinoic acid, based on the fact that the same morphological effect can be produced by putting a small source or retinoic acid on the anterior side of a developing chick wing bud. It has now been shown that this twinning can also be produced by a source of the sonic hedgehog protein.
Thus the anterior-posterior axis of limb bud development is now believed to be controlled by a gradient of sonic hedge hog protein. The evidence convinces me that this is at least part of the explanation.
---------------------
The Dorso-Ventral axis of limb development can be reversed by peeling off the skin, and grafting it back upside down. Several genes homologous to well-known fly genes cause this axis.
Organization of fingers depends in part on certain hox genes:
Specifically hoxD 9, 10, 11, 12, 13
